Combination Reaction: The Formation of Calcium Hydroxide
A combination reaction (also known as a synthesis reaction) is a type of chemical change where two or more substances combine to form a single product. These reactions are common in daily life and often release energy in the form of heat or light.
Theory for Class 10 Science
In this experiment:
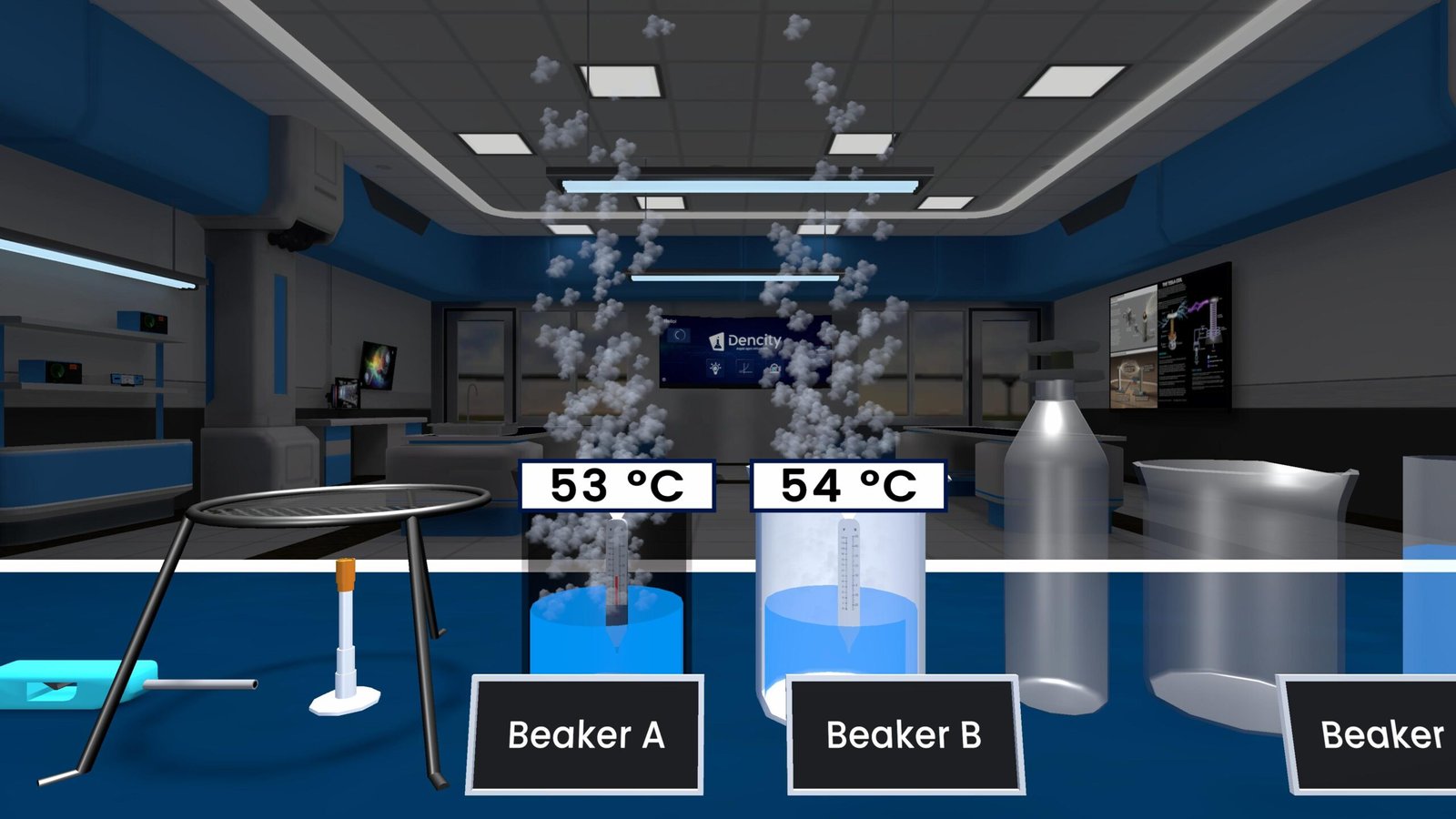
- A small amount of calcium oxide (CaO) is placed in a beaker.
- Water (H₂O) is slowly added to the beaker.
- A vigorous reaction takes place, forming calcium hydroxide (Ca(OH)₂).
- This reaction releases a large amount of heat, making it exothermic.
- A thermometer placed in the beaker shows a rapid rise in temperature.
The chemical equation for the reaction is:
CaO (solid) + H₂O (liquid) → Ca(OH)₂ (aqueous) + Heat
This is a perfect example of a combination reaction—two reactants forming a single product with energy release.
Real-Life Applications
- Construction industry: Used to make cement and mortar.
- Whitewashing: Calcium hydroxide gives walls a bright, clean finish.
- Agriculture: Helps to neutralize acidic soils, improving plant growth.
Observations from the Experiment
- The beaker gets hot due to heat release.
- Temperature rises quickly, proving the reaction is exothermic.
- A white, milky solution (Ca(OH)₂) forms—commonly known as limewater.
Reaction Table
| Reactants | Products | Type of Reaction |
|---|---|---|
| CaO + H₂O | Ca(OH)₂ + Heat | Combination |
Learn Combination Reactions with Dencity
With the Dencity virtual lab, students can safely simulate the reaction between calcium oxide and water. They can observe:
- Heat generation
- Temperature rise
- Formation of limewater
This topic is part of the Class 10 Science curriculum and available on Android, iOS, and desktop via the Dencity app.
Dencity allows you to interact with chemicals safely, test variables, and observe real-time outcomes—making learning engaging and impactful.
Dencity for Teachers
Bring interactive teaching to your classroom with Dencity:
- Simulate combination reactions without the hazard of heat or chemical spills.
- Assign homework with auto-evaluation.
- Use built-in drawing tools for molecular diagrams and observations.
- Let students explore reaction behavior with guided and unguided simulations.
Perfect for Smart Panels and Interactive Screens
Dencity is designed for touch-based classroom panels, allowing teachers and students to perform and observe experiments hands-on—just by tapping and sliding.
Book a Demo or Get Custom Pricing
Interested in transforming your classroom into a virtual science lab? Contact us now for a personalized demo and custom pricing options suited to your school’s needs.
Frequently Asked Questions
- What is a combination reaction?
It’s a reaction where two or more substances combine to form one product. - Why does the beaker get hot?
Because the reaction is exothermic—it releases heat. - What is formed in this experiment?
Calcium hydroxide (Ca(OH)₂), also called limewater. - What is the use of Ca(OH)₂?
In whitewashing, construction, and soil treatment. - Can I simulate this in the Dencity app?
Yes, it is fully simulated with temperature feedback. - Is this experiment safe in real life?
It involves vigorous heat release—better to simulate in Dencity. - Which class is this experiment for?
This is part of the Class 10 Science syllabus. - Can students perform this as homework?
Yes, with auto-assigned tasks and feedback features. - Does Dencity provide real-time reaction visuals?
Absolutely! You can watch the entire process unfold step-by-step. - How can my school get started with Dencity?
Reach out for a customized demo and pricing.