Displacement Reaction: Iron Nails and Copper Sulfate
A displacement reaction is a chemical process in which a more reactive element displaces a less reactive element from its compound. This is also called a substitution reaction. These reactions are driven by the reactivity series of metals, and are often used in industrial and scientific applications.
Theory for Class 10 Science
In this experiment:
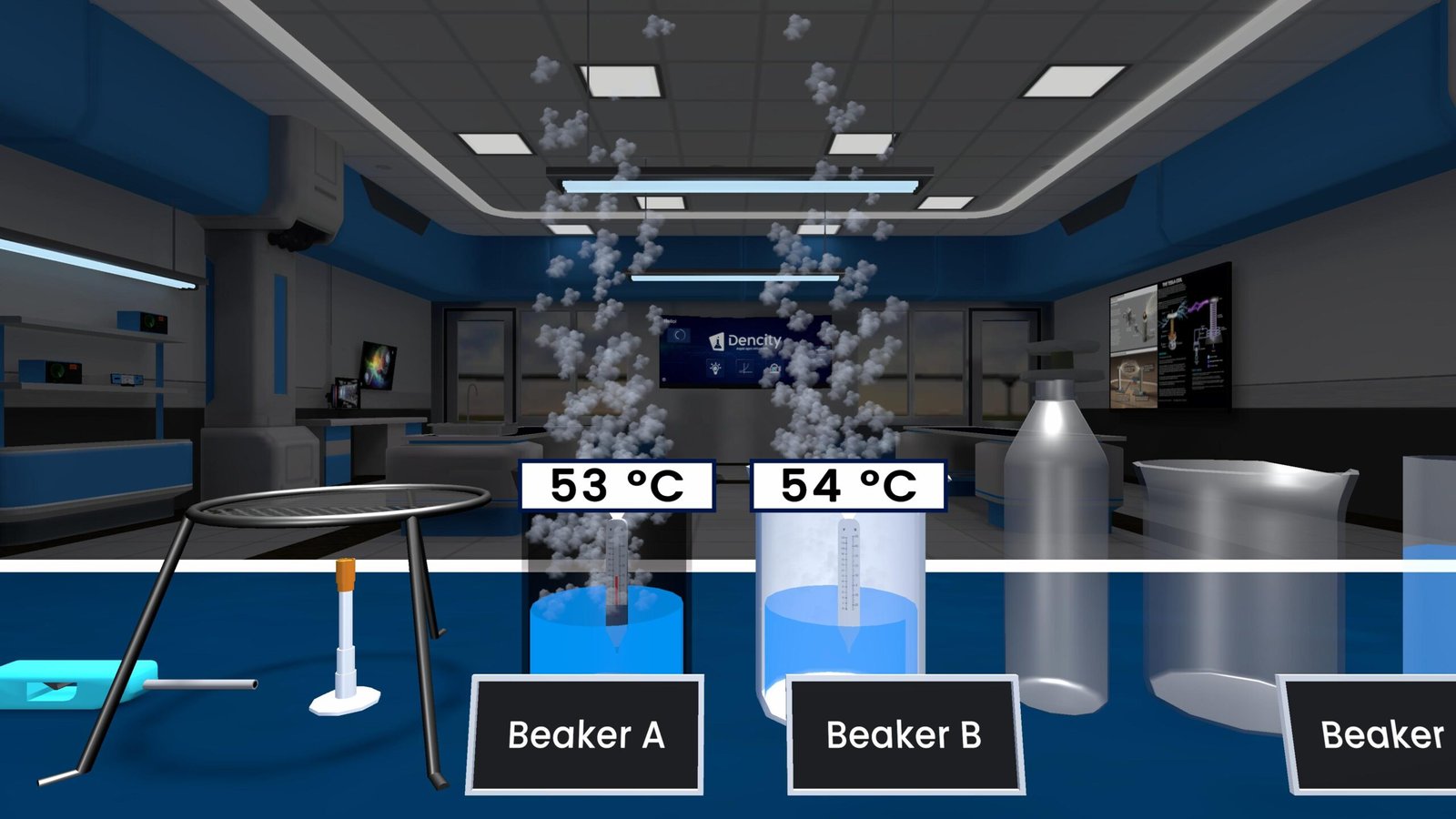
- Two test tubes are filled with copper(II) sulfate solution (CuSO₄).
- An iron nail (Fe) is dipped into one test tube, while the other serves as a control.
- Iron is more reactive than copper, so it displaces copper from its compound.
The reaction is:
Fe + CuSO₄ → FeSO₄ + Cu
Here’s what happens:
- Iron (Fe) replaces copper (Cu) in copper sulfate (CuSO₄).
- This forms iron sulfate (FeSO₄), and copper is deposited on the nail.
Observations from the Experiment
- A reddish-brown layer of copper forms on the iron nail.
- The blue color of the copper sulfate solution fades.
- The solution turns pale green, indicating the formation of iron(II) sulfate.
- In the control setup (without the nail), the solution remains blue, confirming no reaction occurred.
Chemical Equation
Fe + CuSO₄ → FeSO₄ + Cu
This reaction clearly demonstrates metal reactivity and how one metal can replace another in a compound.
Real-Life Applications
- Electroplating: Using displacement to coat objects with metals.
- Metal purification: Removing less reactive metals from ores.
- Galvanic cells: Generating electricity through redox and displacement reactions.
Summary Table
| Case | Observation |
|---|---|
| Fe + CuSO₄ | Copper deposits on iron |
| Fe + CuSO₄ | Solution turns green |
| CuSO₄ only | No color change |
Abbreviations
- Fe – Iron
- Cu – Copper
- CuSO₄ – Copper(II) Sulfate
- FeSO₄ – Iron(II) Sulfate
Visualize Displacement Reactions with Dencity
The Dencity virtual lab lets students conduct the displacement reaction experiment safely and interactively. They can:
- Dip a virtual iron nail into copper sulfate.
- Watch the copper deposit form.
- Observe the color change of the solution.
- Compare with the control sample—all in real time!
This is part of the Class 10 Science syllabus and is available on Android, iOS, and desktop.
Dencity for Teachers
Teachers can elevate interactive teaching with Dencity:
- Assign displacement reaction simulations as homework or in-class activities.
- Use real-time visuals and auto-evaluation tools.
- Engage students in live virtual classrooms.
- Encourage students to explore how the reactivity series affects reactions.
Interactive Panel Ready
Dencity works perfectly with interactive smart panels, allowing touch-based control for conducting, observing, and analyzing chemical reactions.
Contact for Demo and Custom Pricing
Bring Dencity into your classrooms today. Contact us now for a free demo and custom pricing for your school or institution.
Frequently Asked Questions
- What is a displacement reaction?
When a more reactive element replaces a less reactive one in a compound. - Why does copper form on the nail?
Because iron displaces copper from copper sulfate. - What causes the color change?
Copper sulfate (blue) becomes iron sulfate (green) as iron replaces copper. - Is this experiment safe in real life?
It’s safe with precautions, but Dencity offers a virtual and risk-free method. - Is this included in Class 10 Science?
Yes, it’s part of the standard curriculum. - Can I perform this experiment on my phone?
Yes, Dencity is available on Android, iOS, and desktops. - Can teachers assign this as homework?
Absolutely—with auto-evaluation and performance tracking. - Does Dencity explain the steps?
Yes, with clear, step-by-step guidance and interactive visuals. - How is this used in real life?
In metal extraction, battery cells, and coating metals via electroplating. - How can schools get Dencity?
Contact us for a demo and pricing tailored to your needs.