Corrosion: Rusting of Iron – A Class 10 Science Concept
Rusting is a common example of corrosion, which is the gradual destruction of materials—especially metals—due to chemical reactions with their environment. Specifically, rusting refers to the corrosion of iron when it reacts with oxygen and water, forming a reddish-brown flaky substance called iron oxide or rust. Over time, this weakens the metal, making it brittle and fragile.
Understanding the Rusting Process
Rusting occurs only when iron is exposed to both air (oxygen) and moisture (water). The chemical reaction that starts this process is:
(4Fe) + (3O₂) + (6H₂O) → 4Fe(OH)₃ (ferric hydroxide)
This ferric hydroxide then oxidizes further into hydrated ferric oxide, which is commonly known as rust.
Rusting Is an Electrochemical Reaction
- Anode (Iron): Loses electrons (oxidation).
- Cathode (Oxygen): Gains electrons (reduction).
- Electrolyte (Water): Enables ion transfer that drives the reaction.
Factors Affecting Rusting
- Presence of Electrolytes: Salt or acids increase conductivity and speed up rusting.
- Humidity: High moisture levels enhance the rusting process.
- Temperature: Warm conditions accelerate chemical reactions.
- Air Pollution: Gases like carbon dioxide and sulfur dioxide lead to acid rain, which promotes rusting.
Preventing Rusting of Iron
- Painting: Blocks air and moisture.
- Oiling/Greasing: Creates a protective layer.
- Galvanization: Coating iron with zinc, which corrodes instead of iron.
- Alloying: Using stainless steel by adding chromium and nickel.
- Desiccants: Using silica gel to keep the environment dry.
- Electroplating: Adding a layer of another metal.
- Cathodic Protection: Connecting iron to a more reactive metal which sacrifices itself.
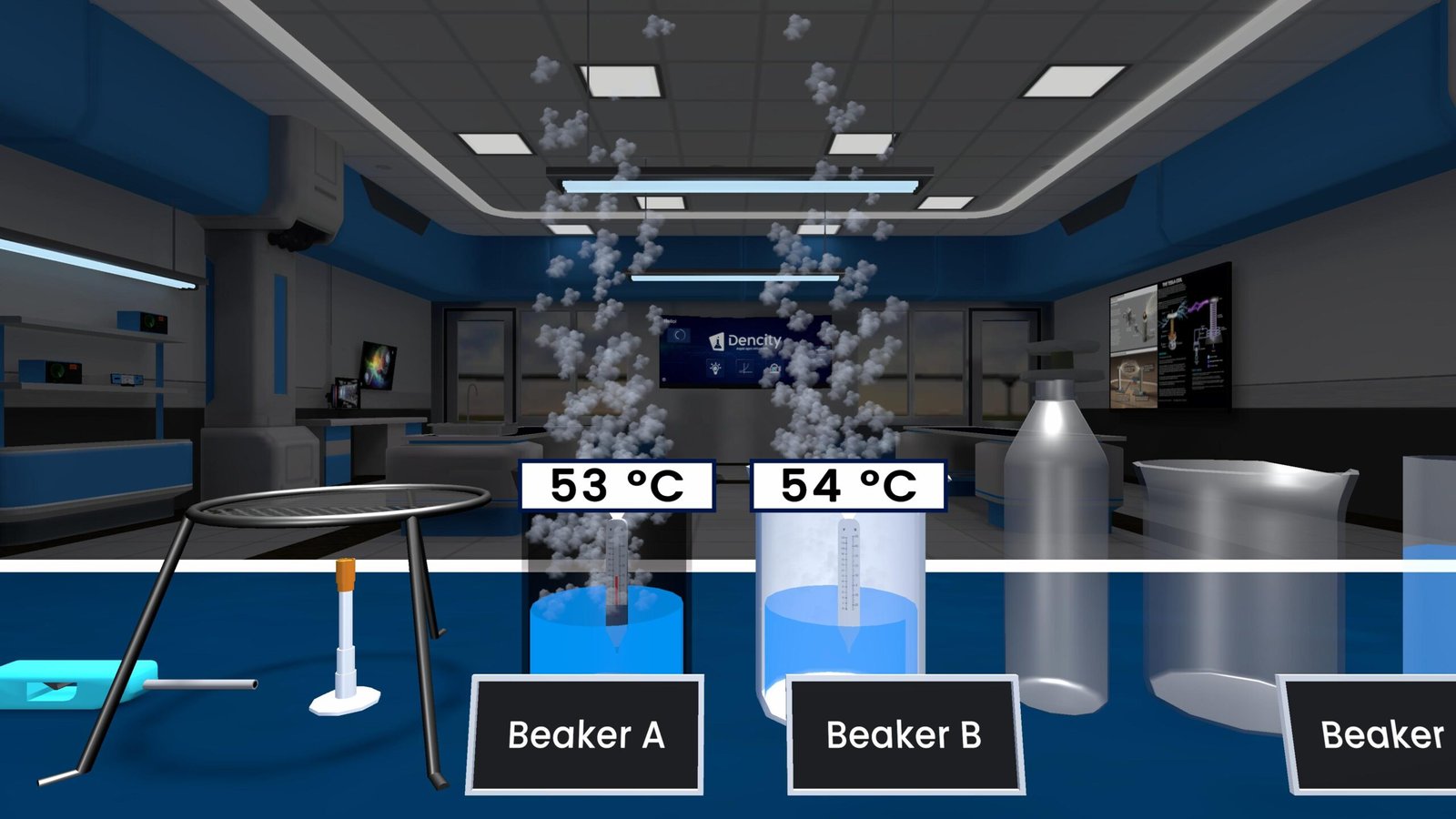
Observations from a Rusting Experiment
- Test Tube A (Air + Water): The nail rusted both where it touched air and water.
- Test Tube B (Water + Oil Layer): No rust because oil blocked the air.
- Test Tube C (Dry Air + Calcium Chloride): No rust due to the absence of moisture.
This confirms that both oxygen and moisture are essential for rusting to occur.
Simplified Chemical Equation
Fe + O₂ + H₂O → Fe(OH)₃
Fe(OH)₃ → Fe₂O₃·xH₂O (rust)
Explore Rusting of Iron with Dencity – A Virtual Science Lab for Class 10
With Dencity, students can safely and affordably explore the Rusting of Iron Experiment virtually. It is part of our class 10 science curriculum and provides a realistic, interactive experience of how rusting works and how different conditions affect it.
Dencity allows students to:
- Simulate different environmental conditions.
- Visually observe where rusting happens and why.
- Learn with step-by-step guidance and real-time feedback.
- Understand the importance of science experiments in daily life.
Dencity is available on Android, iOS, and Desktop, making it easy for students to learn anytime, anywhere.
Dencity for Teachers
Interactive Teaching becomes a reality with Dencity’s classroom features:
- Create virtual classrooms to perform live experiments.
- Assign interactive science experiments with a single click.
- Use real-time simulations to explain the theory behind rusting.
- Track student performance and engagement effortlessly.
Optimized for Interactive Touch Panels
Dencity is fully compatible with interactive touch panels used in modern classrooms. Teachers and students can manipulate experiments with simple touch gestures, enabling better engagement and deeper understanding.
For Schools and Educational Institutions
Want to bring Dencity to your school? Contact us today for a customized pricing plan or to request a demo tailored for your institution’s needs.
10 Frequently Asked Questions
- What is rusting?
Rusting is the corrosion of iron when it reacts with air and water, forming a reddish substance. - Why does rusting occur?
Because iron reacts with oxygen and moisture in an electrochemical process. - Is rusting harmful?
Yes, it weakens the iron, making it unsafe for use. - Can rusting happen without water?
No, both water and air are required for rusting to occur. - What speeds up rusting?
Salt, high humidity, warmth, and pollution. - How can we stop rusting?
Using paint, oil, galvanization, or other protective methods. - What is galvanization?
Coating iron with zinc to protect it from rusting. - What is the role of calcium chloride in the experiment?
It absorbs moisture to create a dry environment, preventing rusting. - Why did the nail not rust in oil-covered water?
Oil acted as a barrier, preventing oxygen from reaching the water. - How does Dencity help in learning rusting?
Dencity provides a virtual, interactive platform to experiment safely and understand rusting through simulations.
For more information, explore the Dencity App and bring interactive learning to your classroom today.