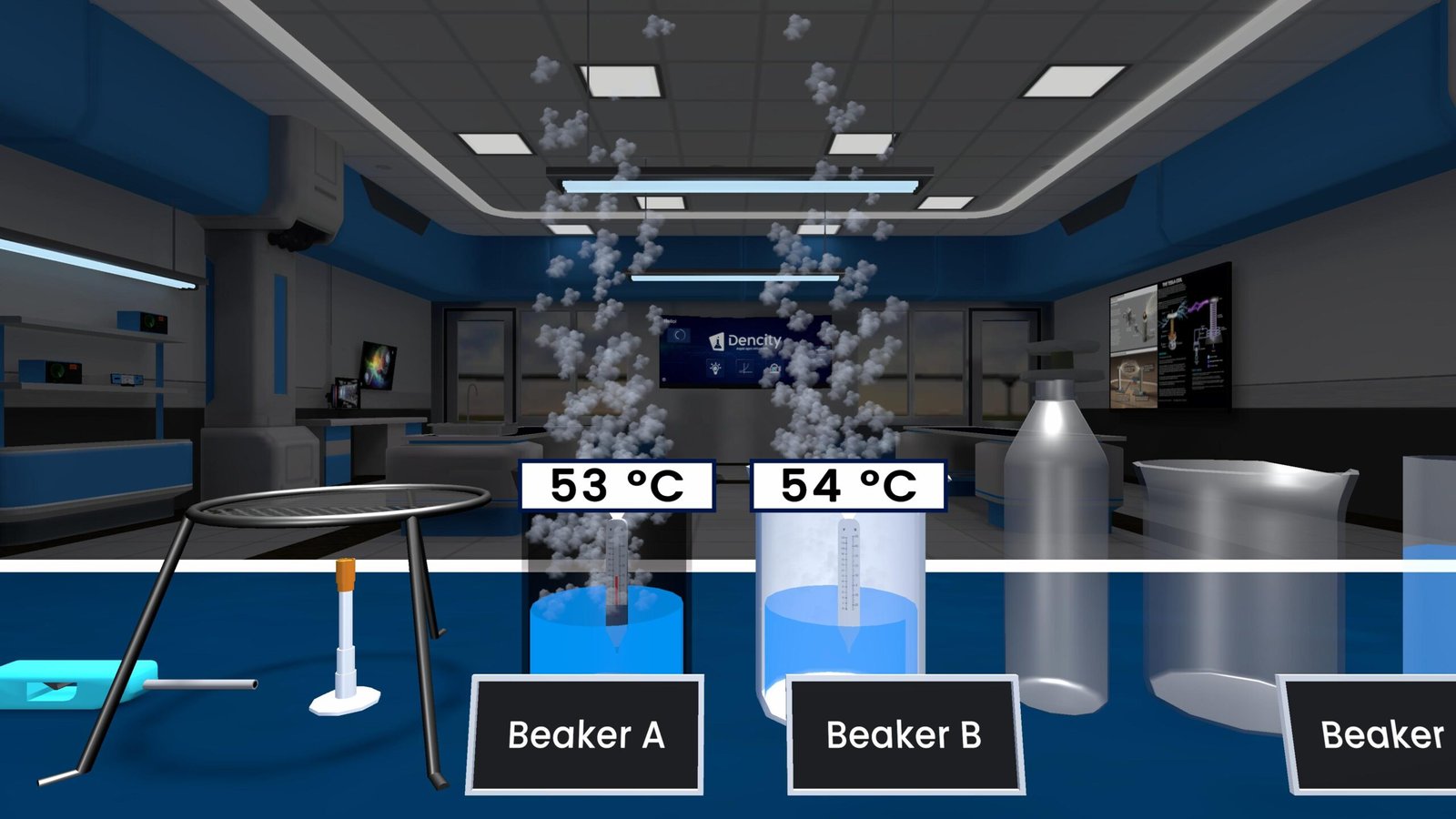
Reaction Between Metal and Acid: Formation of Salt and Hydrogen Gas
A metal-acid reaction is a fundamental chemical process where a metal reacts with an acid to form a salt and hydrogen gas. This is a classic single displacement reaction, where the metal replaces hydrogen in the acid.
Theory for Class 10 Science
When a reactive metal like zinc (Zn) is added to an acid:
- A salt is formed (depending on the acid used).
- Hydrogen gas (H₂) is released.
- The reaction is exothermic, meaning it releases heat.
- The hydrogen gas forms bubbles, which can be tested for by collecting them in soap water.
Reactions with Common Acids:
- Zinc + Sulphuric Acid:
Zn + H₂SO₄ → ZnSO₄ + H₂ ↑ - Zinc + Hydrochloric Acid:
Zn + 2HCl → ZnCl₂ + H₂ ↑ - Zinc + Acetic Acid:
Zn + 2CH₃COOH → (CH₃COO)₂Zn + H₂ ↑
When these bubbles are exposed to a burning matchstick, they burn with a faint blue flame, confirming the presence of hydrogen gas.
Real-Life Applications
- Hydrogen production in laboratories.
- Demonstrates the reactivity of metals and acids.
- Used in chemistry education to illustrate displacement and exothermic reactions.
- Tests the flammability of hydrogen with the characteristic “pop” sound.
Observations from the Experiment
- Zinc reacts rapidly with acids.
- Strong acids like H₂SO₄ and HCl produce vigorous bubbling.
- Weak acids like acetic acid react more slowly.
- Hydrogen gas travels through a tube into a soap solution and forms bubbles.
- When ignited, the gas burns with a faint blue flame.
Summary Table
| Item | Role | Effect |
|---|---|---|
| Zn | Metal | H₂ gas forms |
| H₂SO₄ | Strong acid | Vigorous reaction |
| HCl | Strong acid | Vigorous reaction |
| CH₃COOH | Weak acid | Slow reaction |
| H₂ | Pop sound | Burns with blue flame |
Try This Experiment in the Dencity App
The Dencity virtual lab lets students safely explore metal-acid reactions:
- Choose between different acids and metals.
- Observe gas formation, soap bubbles, and flame test virtually.
- Compare reactions of strong and weak acids in real-time.
This experiment is a part of the Class 10 Science curriculum and is fully accessible on Android, iOS, and desktops through the Dencity app.
Dencity for Teachers
Dencity makes interactive teaching simple and effective:
- Perform virtual metal-acid reactions without safety risks.
- Let students observe hydrogen tests using animated simulations.
- Assign interactive experiments with auto-evaluation and progress tracking.
- Use annotation tools to guide learners step-by-step.
Perfect for Touch Panels in Classrooms
Dencity supports interactive smart panels, letting students drag, drop, and ignite elements directly on-screen—perfect for group learning and classroom demos.
Get a Demo or Custom Pricing
Ready to upgrade your science lab? Contact us today to schedule a free demo and receive custom pricing options for your school.
Frequently Asked Questions
- What happens when a metal reacts with an acid?
A salt and hydrogen gas are formed. - What does hydrogen gas do when ignited?
Burns with a faint blue flame and makes a pop sound. - Why does zinc react more with strong acids?
Strong acids release more H⁺ ions, increasing the reaction rate. - What is the gas collected during the reaction?
Hydrogen (H₂). - Can I try this in the Dencity app?
Yes, with safe and interactive simulations. - Is this experiment in the Class 10 syllabus?
Yes, it’s a standard part of Class 10 Science. - How can teachers assess student learning?
Through auto-marked homework and visual participation tracking. - Is it mobile-friendly?
Yes, works on Android, iOS, and desktops. - Do I need lab materials to perform this in Dencity?
No, it’s fully simulated in a virtual environment. - How can schools start using Dencity?
Contact us for a demo and custom implementation support.