Reactivity of Metals with Water: From Fire to Bubbles
When metals react with water, they form metal hydroxides (or oxides, in the case of steam) and release hydrogen gas. The intensity of the reaction depends on the metal’s reactivity—some react explosively, some gently, and others not at all.
Theory for Class 10 Science
Here’s how different metals behave with water:
1. Sodium (Na)
- Violently reactive with cold water.
- Reaction is highly exothermic; hydrogen gas may ignite.
- Sodium floats and moves rapidly on water due to hydrogen evolution.
Reaction:
2Na + 2H₂O → 2NaOH + H₂ + heat
2. Potassium (K)
- Even more reactive than sodium.
- Reaction with cold water is explosive.
- Produces potassium hydroxide and ignites hydrogen gas.
Reaction:
2K + 2H₂O → 2KOH + H₂ + heat
3. Calcium (Ca)
- Reacts moderately with cold water.
- Forms bubbles and floats due to attached hydrogen gas bubbles.
- No fire is observed.
Reaction:
Ca + 2H₂O → Ca(OH)₂ + H₂
4. Iron (Fe)
- Does not react with cold or hot water.
- Reacts only with steam at high temperature.
- Produces iron oxide and hydrogen gas.
Reaction:
3Fe + 4H₂O (steam) → Fe₃O₄ + 4H₂
Real-Life Applications
- Sodium and potassium are stored in kerosene to prevent contact with moisture.
- Calcium hydroxide is used in construction and water treatment.
- Iron-steam reaction is foundational in thermodynamics education.
- Hydrogen gas from these reactions is used as clean fuel and in lab demonstrations.
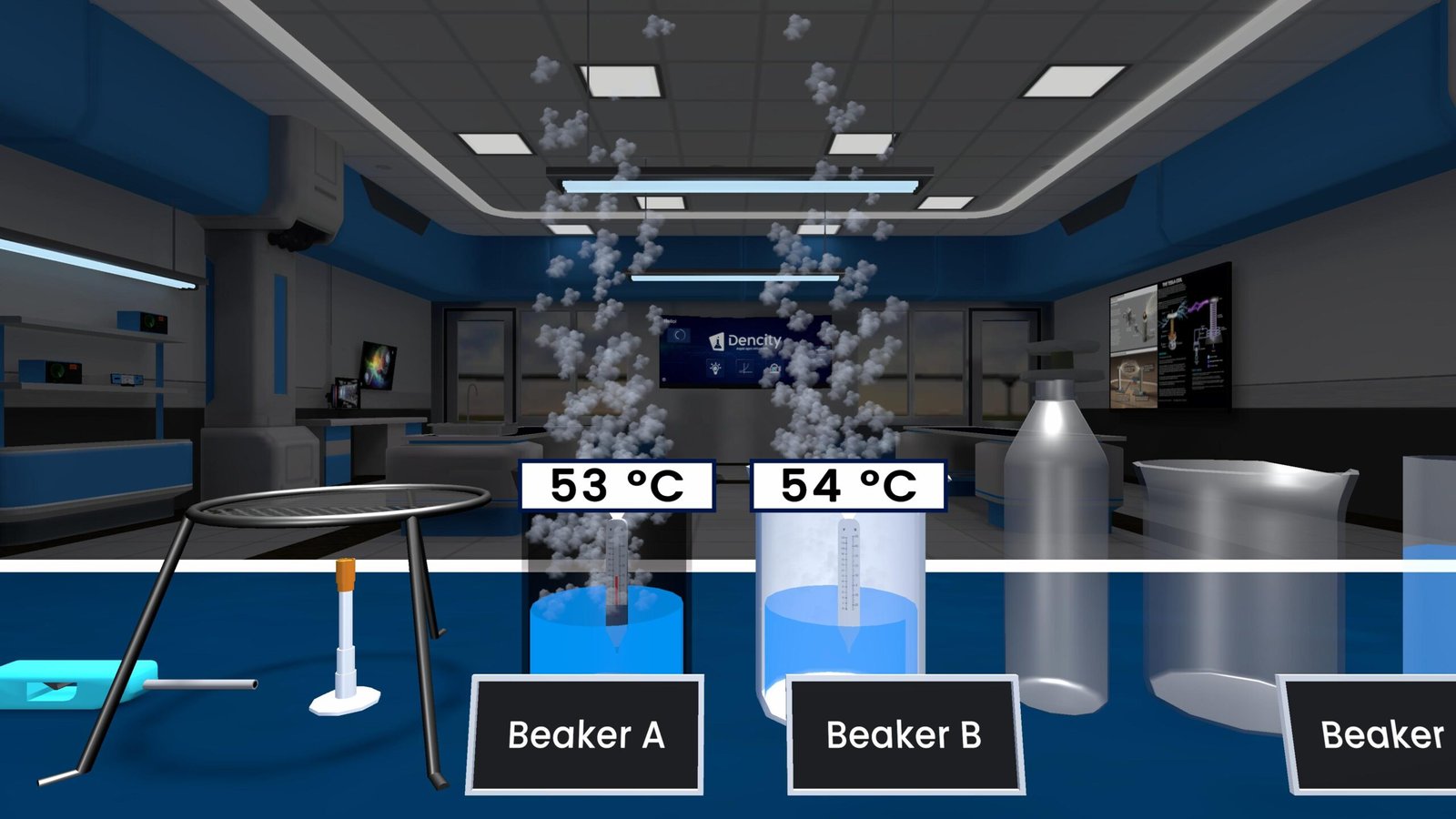
Observations from the Experiment
- Sodium and potassium: Violent, fiery reactions.
- Calcium: Bubbling, gentle reaction, no flame.
- Iron: Only reacts with steam, no action in cold/hot water.
- Hydrogen gas is released in all applicable reactions.
Summary Table
| Metal | Cold Water | Hot Water | Steam |
|---|---|---|---|
| Sodium | ✓ | ✓ | ✓ |
| Potassium | ✓ | ✓ | ✓ |
| Calcium | ✓ | ✓ | ✓ |
| Iron | ✗ | ✗ | ✓ |
Safely Explore Reactivity of Metals with Water with Dencity
The Dencity virtual science lab lets students:
- Simulate reactions of different metals with cold, hot water, and steam.
- Observe hydrogen gas evolution, fire, and movement of metals.
- Compare reactivity trends of metals safely.
This is part of the Class 10 Science curriculum and is accessible on Android, iOS, and desktop using the Dencity app.
Dencity for Teachers
With Dencity, teachers can:
- Conduct safe, virtual demonstrations of hazardous metal reactions.
- Explain hydrogen gas evolution and energy changes visually.
- Track student understanding with auto-grading and real-time feedback.
- Enable interactive simulations for practical chemistry lessons.
Touch-Friendly for Classroom Panels
Dencity supports smart classroom panels, allowing students to:
- Select a metal,
- Add different forms of water (cold, hot, steam),
- Observe immediate and safe reactions with touch gestures.
Request a Demo or Custom Pricing
Want to add powerful virtual chemistry experiments to your science curriculum? Contact us today for a free demo and get custom pricing for your school.
Frequently Asked Questions
- Which metals react violently with water?
Sodium and potassium. - Does calcium react with cold water?
Yes, but less violently than Na or K. - Why is iron stored safely in water?
Because it does not react with cold or hot water, only with steam. - What gas is released in these reactions?
Hydrogen gas (H₂). - Can students simulate this in Dencity?
Yes, with real-time, safe simulations. - Which class covers this topic?
This is part of Class 10 Science. - Is Dencity compatible with phones and desktops?
Yes—available on Android, iOS, and desktop. - Can teachers assign this virtually?
Yes, with auto-evaluation and student tracking. - Are real-life visuals shown in Dencity?
Yes, including hydrogen bubbling, flame, and movement. - How can schools get started?
Contact us to book a demo and explore implementation options.