Carbon Compounds Experiment
Carbon compounds are chemical substances that contain carbon atoms bonded with other elements such as hydrogen, oxygen, nitrogen, sulphur, and halogens like chlorine. These compounds are the foundation of organic chemistry and play a major role in living organisms as well as industrial applications.
Understanding the Theory
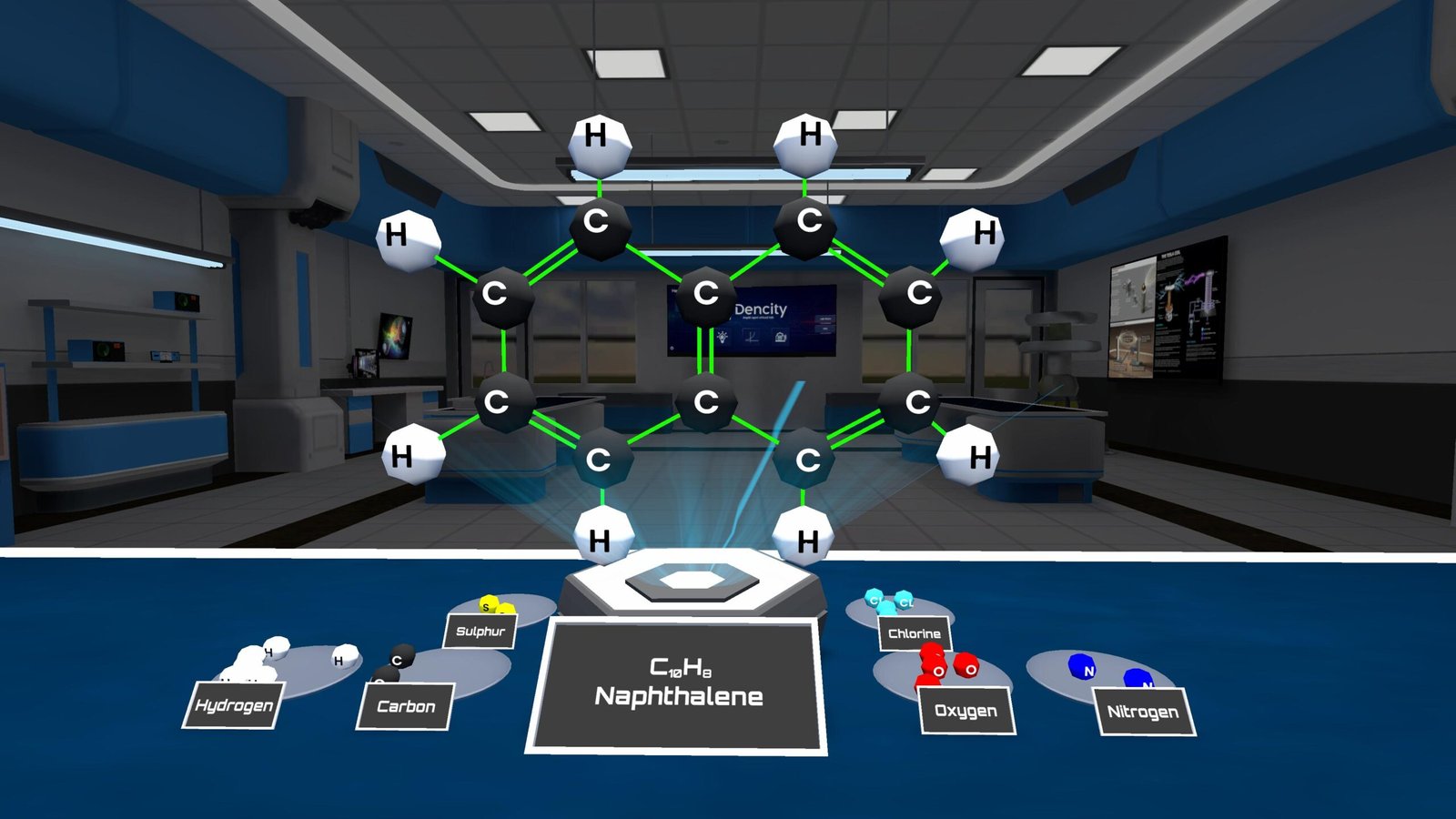
Carbon has a valency of 4, which means it can form four strong covalent bonds. This makes carbon extremely versatile, allowing it to create chains, branches, or rings of atoms. These structures can involve single, double, or triple bonds, giving rise to a wide variety of molecules.
In a molecule builder experiment, we can use:
- 12 carbon atoms
- 14 hydrogen atoms
- 8 oxygen atoms
- 8 nitrogen atoms
- 6 sulphur atoms
- 6 chlorine atoms
Each atom bonds according to its valency:
- Hydrogen (valency 1): forms one bond.
- Oxygen (valency 2): forms two bonds.
- Nitrogen (valency 3): forms three bonds.
- Sulphur (valency 2, 4, or 6): usually forms two bonds in stable organic compounds.
- Chlorine (valency 1): forms one bond, common in haloalkanes.
All of this bonding follows the octet rule, which means atoms combine in such a way that each achieves a full outer shell.
Real-Life Applications
- Carbon compounds form the base of pharmaceuticals, plastics, fuels, and food molecules.
- They are essential in DNA, proteins, and enzymes—the building blocks of life.
- Chlorinated and sulphur-containing compounds are important in industry for modifying reactivity and solubility.
Key Observations
- Increasing carbon atoms increases molecular complexity.
- More hydrogen atoms create saturated hydrocarbons like alkanes.
- Oxygen and nitrogen introduce functional groups such as alcohols and amines.
- Adding chlorine or sulphur changes chemical reactivity and solubility.
- Incorrect bonding (like excess high-valency atoms without partners) leads to unstable molecules.
Summary Table
| Element | Valency | Role in Molecules |
|---|---|---|
| Carbon | 4 | Forms chains and rings |
| Hydrogen | 1 | Completes bonds |
| Oxygen | 2 | Creates double bonds, functional groups |
| Nitrogen | 3 | Found in amines and rings |
| Sulphur | 2 | Forms bonds in thio-compounds |
| Chlorine | 1 | Found in haloalkanes |
Learning with Dencity Virtual Science Lab
With the Dencity app, students can perform the Carbon Compounds Experiment virtually. Instead of just reading theory, learners can actually build molecules and observe how different atoms bond based on their valency. This makes it easier to understand how real-life compounds like alcohols, amines, or plastics are formed.
The Dencity virtual lab allows:
- Safe and cost-effective science experiments without chemicals.
- Interactive learning where students can manipulate atoms and see results in real-time.
- Step-by-step visual explanations that make complex topics simple.
Whether you’re in class 11 science or preparing for higher studies, Dencity bridges the gap between theory and practice.
Dencity for Teachers
For teachers, Dencity promotes interactive teaching by offering:
- Virtual classroom features where experiments can be demonstrated live.
- Real-time control sharing so students can try experiments under supervision.
- Automatic progress tracking and homework assignment.
- Compatibility with interactive touch panels, making classroom teaching highly engaging.
Schools and institutions can also request customized pricing or a demo to integrate Dencity into their science curriculum.
Frequently Asked Questions
1. What are carbon compounds?
Carbon compounds are substances made of carbon bonded with elements like hydrogen, oxygen, nitrogen, sulphur, and chlorine.
2. Why is carbon so versatile in bonding?
Because carbon has four valence electrons, it can form four stable covalent bonds with many elements.
3. What is the octet rule?
The octet rule states that atoms combine in a way that ensures each has eight electrons in its outer shell.
4. How do hydrogen and oxygen contribute to molecules?
Hydrogen completes bonds while oxygen forms double bonds or functional groups like alcohols.
5. What are functional groups?
Functional groups are specific atom arrangements like –OH (alcohols) or –NH2 (amines) that define a molecule’s chemical properties.
6. Why are carbon compounds important in biology?
They form DNA, proteins, fats, and carbohydrates—the essential molecules of life.
7. What is the difference between saturated and unsaturated carbon compounds?
Saturated compounds (alkanes) have only single bonds, while unsaturated compounds (alkenes, alkynes) contain double or triple bonds.
8. How does chlorine affect carbon compounds?
Chlorine atoms form haloalkanes, which are used in solvents, medicines, and industrial processes.
9. Can Dencity help in understanding molecular structures?
Yes, Dencity allows students to visualize and build molecules, making abstract concepts easy to understand.
10. On which devices can Dencity be used?
The Dencity app works on Android, iOS, and desktop platforms, and is also optimized for interactive touch panels in classrooms.