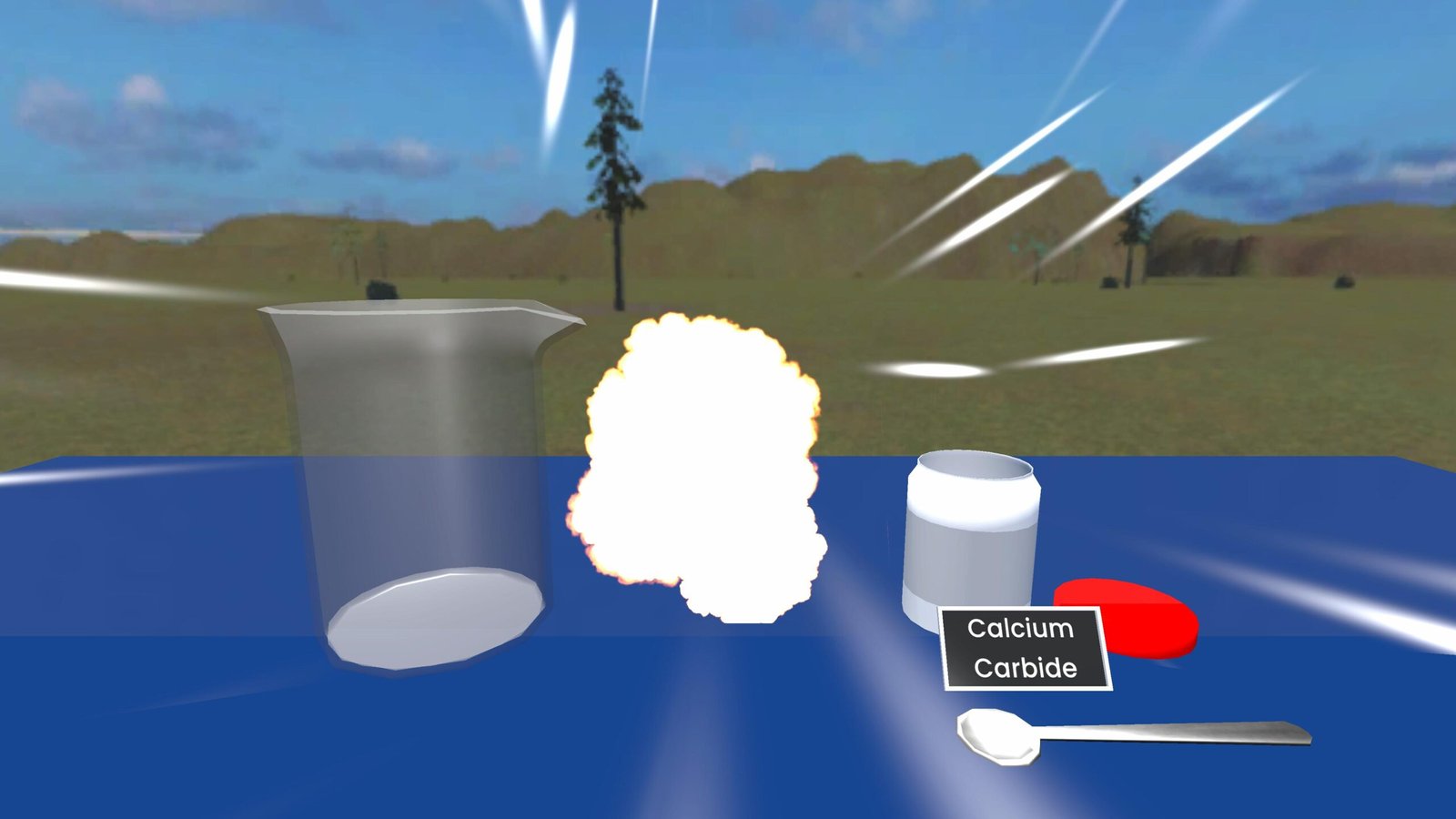
Calcium Carbide and Water — Acetylene Gas Reaction (Experiment)
Class: class 12 science
Definition:
When calcium carbide (CaC2) reacts with water (H2O), it produces acetylene gas (C2H2) and calcium hydroxide (Ca(OH)2). This reaction releases heat (exothermic) and generates a highly flammable gas.
Chemical equation (plain text):
CaC2 + 2 H2O → C2H2 + Ca(OH)2
What this experiment shows (detailed explanation)
This experiment demonstrates how a chemical reaction between a solid and water can rapidly produce a gas and heat. Calcium carbide is a greyish-black solid that reacts immediately with water. The solid’s surface reacts to form acetylene gas, which is highly flammable, and calcium hydroxide, a soluble base. Because the gas forms quickly, if the reaction happens in a confined space the pressure increases — which makes the experiment potentially dangerous.
Different amounts of water and different sealing conditions change the outcome:
- If very little water is added, the reaction is negligible and there may be no visible effect.
- If the container is half-filled with water and sealed, gas forms and pressure builds; the container may bulge.
- If the container is filled with water but left open, the gas escapes and no pressure builds up.
- If the container is full of water and sealed, a large volume of gas is produced and pressure can build to the point the container bursts — this can cause an explosion and is hazardous.
Key scientific principles illustrated: reaction stoichiometry, exothermic reactions (heat release), gas generation and behavior, and gas laws (pressure build-up in confined spaces). This experiment is a clear demonstration of cause (adding water) and rapid effect (gas + heat), and it highlights why controlling reaction conditions and containment is critical in chemistry.
Safety note (important): This reaction produces a flammable gas (acetylene) and can generate pressure and heat. It should never be carried out in an uncontrolled or improvised container, and must only be performed by trained personnel in a proper laboratory with appropriate protective equipment and ventilation. Do not attempt sealed‑container demonstrations with this reaction outside of supervised, safety‑equipped labs.
Real-life uses and relevance
- Miner’s lamps: Historically, acetylene produced from calcium carbide was used for portable lighting.
- Welding and cutting: Acetylene is used in oxy-acetylene torches for cutting and welding metals.
- Education and demonstrations: Under controlled lab conditions, the reaction illustrates exothermic reactions, gas production, and effects of confinement on pressure.
- Industrial awareness: Understanding such gas-producing reactions is important for lab safety, chemical handling, and emergency planning.
Observations (plain-language)
- Increasing the amount of water generally increases the amount of acetylene gas produced (up to limits set by the amount of CaC2).
- Sealing the reaction container leads to pressure buildup; leaving it open lets gas escape safely (but still flammable).
- Very small amounts of water produce little or no visible reaction.
- A sealed, full container can burst due to rapid pressure rise — this is dangerous.
Summary Table
| Water Level | Container Seal | Outcome |
|---|---|---|
| Very low | Open / Closed | No visible reaction |
| Half-filled | Sealed | Bottle/container expands (pressure increase) |
| Full | Open | Gas escapes (no pressure build-up) |
| Full | Sealed | Dangerous pressure build-up — container may burst |
Why teach this using the Dencity virtual science lab?
This reaction is hazardous to run physically for many schools. The Dencity virtual science lab lets students explore the same chemical principles — reaction products, heat release, gas generation, and effects of confinement — in a safe, cost-efficient, and interactive environment. Using the Dencity app (available on Android, iOS, and Desktop), learners can:
- Simulate adding controlled amounts of water to CaC2 and observe gas volume, temperature change, and pressure in real time.
- Change variables (amount of CaC2, water added, container openness) and immediately see the results without risk.
- Visualize the chemical equation and step-by-step reaction outcomes with clear, student-friendly explanations.
- Practice experimental design and safety planning (for example, testing “what if” scenarios) without exposing anyone to real hazards.
- Access virtual safety checks that show why sealed-container demonstrations are dangerous and how to mitigate risk.
Because the Dencity virtual lab reproduces realistic physics and chemistry behavior, students build correct intuition about dangerous reactions while staying safe.
Dencity for Teachers
Dencity supports interactive teaching and classroom efficiency:
- Interactive demonstrations: Run live simulations of the CaC2 + H2O reaction in front of the class without handling hazardous chemicals.
- Assign and assess: Create and assign virtual lab tasks quickly; students submit results and teachers get automatic reports on understanding and performance.
- Safety-first pedagogy: Use the simulator to teach lab safety, risk assessment, and proper handling procedures conceptually.
- Customizable variables: Let students experiment with parameters (amounts, container type, venting) to learn experimental design and consequences.
- Remote & hybrid-ready: Conduct synchronous or asynchronous lab sessions — ideal for remote learning or mixed classrooms.
- Interactive learning: Encourage students to predict outcomes, test hypotheses in the simulator, and receive immediate feedback — boosting engagement and retention.
Works well on interactive touch panels
The Dencity app is optimized for interactive touch panels and large classroom displays. Teachers can manipulate variables with touch gestures, zoom into reaction visualizations, highlight safety steps, and run group activities that make the science lab experience truly interactive and hands-on — without the hazards and costs of real chemicals.
Contact for demo or institutional pricing
Educational institutions interested in integrating Dencity virtual lab into their curriculum can request a demo or ask for customized pricing. Visit dencityapp.in or contact the Dencity team to arrange a school trial, enterprise account, or on-site presentation.
Frequently Asked Questions (FAQs)
- What exactly happens when calcium carbide meets water?
Calcium carbide reacts with water to form acetylene gas (C2H2) and calcium hydroxide (Ca(OH)2); the reaction releases heat and gas rapidly. - Is acetylene dangerous?
Yes. Acetylene is highly flammable and can form explosive mixtures with air; it must be handled with strict safety measures. - Why can a sealed container burst during this reaction?
Rapid gas production increases internal pressure. If the container cannot safely vent, the pressure may exceed its strength and cause rupture. - Can students perform this experiment safely in school labs?
Not without specialized equipment, strict supervision, and safety systems. For most schools, a virtual simulation (like Dencity) is the safer option. - What real-world uses does acetylene have?
Acetylene is widely used in oxy-acetylene welding and cutting, and historically for miner’s lamps and lighting. - How does varying the amount of water affect results?
More water (up to stoichiometric limits) generally increases the rate and total amount of gas produced; reaction conditions also affect heat and pressure. - Why teach this experiment virtually?
The virtual lab demonstrates core chemical and physical principles while eliminating the risk of fire, explosion, and toxic exposure — making learning safer and more accessible. - Does Dencity show numerical data (pressure, temperature, gas volume)?
Yes. Dencity’s simulations include real-time numerical readouts and graphs so students can analyze quantitative effects of variable changes. - Can teachers customize the simulation for different learning levels?
Yes. Teachers can adjust complexity, add guided steps for younger students, or enable advanced controls for higher secondary (class 12) learners. - How can my school get a demo or pricing details?
Visit dencityapp.in to request a demo, discuss licensing, or get customized institutional pricing.
Bring advanced chemistry concepts into your classroom safely and effectively — use Dencity virtual lab to teach hazardous reactions like the calcium carbide and water reaction without real-world risk.