Thanks! Based on your line “Carbon dioxide passing through lime water (Decomposition Reaction)”, it sounds like you’re referring to the classic lime water test for carbon dioxide, which is often associated with decomposition reactions, especially the thermal decomposition of calcium carbonate (CaCO₃) into calcium oxide (quicklime) and carbon dioxide gas.
Let me now generate a full SEO-optimized and student-friendly content article for this experiment based on the structure you’ve asked for.
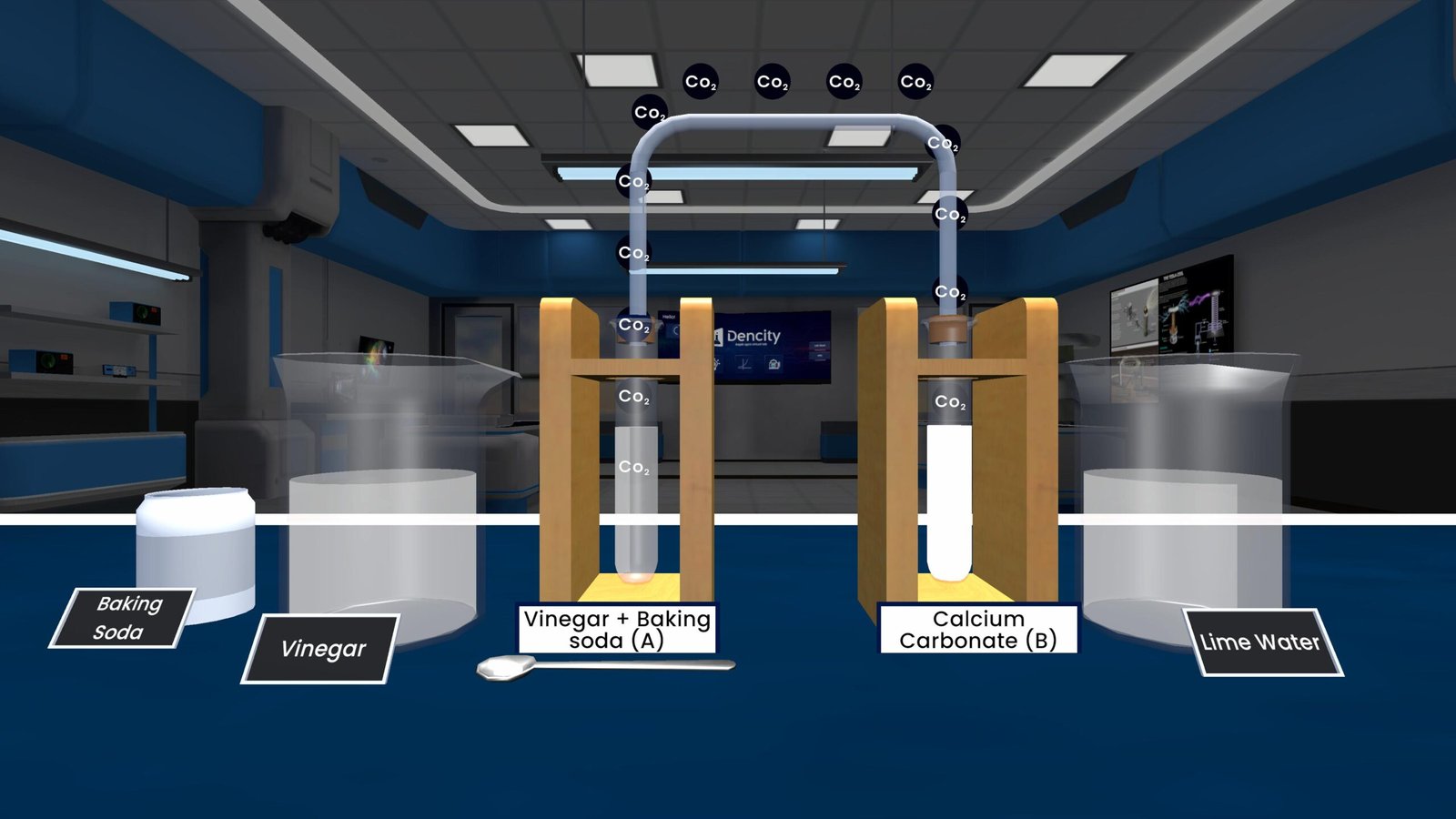
Carbon Dioxide Passing Through Lime Water – Decomposition Reaction Experiment
One of the most important and visually clear experiments in chemistry is observing carbon dioxide reacting with lime water. This experiment not only teaches us about chemical reactions, but also shows the evidence of a gas being formed during a decomposition reaction.
This experiment is simple, effective, and deeply educational—perfect for school science labs and virtual simulations.
What is Happening in This Experiment?
In this experiment, we thermally decompose calcium carbonate (CaCO₃)—commonly found in limestone—by heating it. When heated, it breaks down into calcium oxide (CaO) and carbon dioxide (CO₂):
Calcium carbonate → Calcium oxide + Carbon dioxide
(CaCO₃ → CaO + CO₂)
The carbon dioxide gas released is then bubbled through lime water, which is a dilute solution of calcium hydroxide (Ca(OH)₂). As CO₂ passes through lime water, a white precipitate of calcium carbonate forms, turning the lime water milky:
Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water
(Ca(OH)₂ + CO₂ → CaCO₃ + H₂O)
If you keep passing more CO₂ through the lime water, the milky color disappears. That’s because the calcium carbonate further reacts to form soluble calcium hydrogen carbonate:
Calcium carbonate + Carbon dioxide + Water → Calcium hydrogen carbonate
(CaCO₃ + CO₂ + H₂O → Ca(HCO₃)₂)
Why Is This a Decomposition Reaction?
- Decomposition means breaking a compound into simpler substances.
- Here, calcium carbonate breaks down into calcium oxide and carbon dioxide upon heating.
- This experiment clearly shows how thermal decomposition works.
Real-Life Importance of This Reaction
- This reaction is used in the lime industry to make quicklime.
- The lime water test is used in labs to detect the presence of carbon dioxide.
- It’s part of the carbon cycle, important in nature and environmental science.
Key Observations
- Heating CaCO₃ produces gas (CO₂).
- Passing CO₂ through lime water turns it milky.
- Passing excess CO₂ makes the milkiness disappear.
Summary Table
| Step | Observation |
|---|---|
| Heating calcium carbonate | Gas is released (CO₂) |
| CO₂ passed through lime water | Lime water turns milky (CaCO₃ formed) |
| Excess CO₂ passed | Milky solution clears up (Ca(HCO₃)₂) |
Perform This Experiment Safely with Dencity
With the Dencity Virtual Science Lab, you can explore the Carbon Dioxide and Lime Water experiment safely and interactively on your device.
Whether you’re in Class 10 Science or preparing for higher classes, this experiment helps you visualize a decomposition reaction, observe gas behavior, and understand chemical changes in real time—without any need for flames or chemicals.
Dencity for Teachers
Dencity supports interactive teaching by enabling:
- Virtual classrooms with hands-on student activities
- Safe experimentation without needing lab infrastructure
- Step-by-step observation simulations
- Quick homework assignments and automatic evaluations
Perfect for explaining concepts like thermal decomposition, gas detection, and precipitation reactions.
Touch-Enabled for Classroom Smart Panels
The Dencity app runs beautifully on interactive touch panels, making it ideal for live classroom demonstrations. Teachers can manipulate the experiment with touch and explain the outcomes in real-time to the entire class.
Schools – Contact Us for a Demo
Want to integrate virtual science experiments into your school curriculum?
Contact us for a free demo or to learn more about custom pricing for your school or institution.
Explore science the modern, interactive, and cost-effective way!
Frequently Asked Questions
- What is lime water?
It is a dilute solution of calcium hydroxide, used to test for carbon dioxide. - Why does lime water turn milky?
Because carbon dioxide reacts with calcium hydroxide to form insoluble calcium carbonate, a white precipitate. - What is thermal decomposition?
A chemical reaction where heat breaks down a compound into simpler substances. - Why does the milkiness disappear after a while?
Due to the formation of soluble calcium hydrogen carbonate. - Which gas is tested using lime water?
Carbon dioxide (CO₂). - Which class is this experiment from?
This is commonly taught in Class 10 Science. - Can I do this experiment on the Dencity app?
Yes! It is fully available as a virtual simulation on the app. - Is this experiment dangerous in real life?
Slightly—since it involves heating and gas handling. Dencity removes all risks by simulating it virtually. - Can teachers assign this as homework?
Yes. Teachers can assign it instantly and track completion. - How can I get access to Dencity?
Visit dencityapp.in to get the app and request a demo.
Let me know if you’d like the same content formatted for WordPress or as part of a batch upload.