Decomposition Reaction: Breaking Down Compounds with Heat
A decomposition reaction is a chemical reaction where one compound breaks down into two or more simpler substances. This process usually requires energy in the form of heat, light, or electricity, making it an endothermic reaction.
These reactions are common in both chemical labs and industrial applications, especially in processes like metal extraction, cement production, and airbag deployment.
Theory for Class 10 Science
In this experiment:
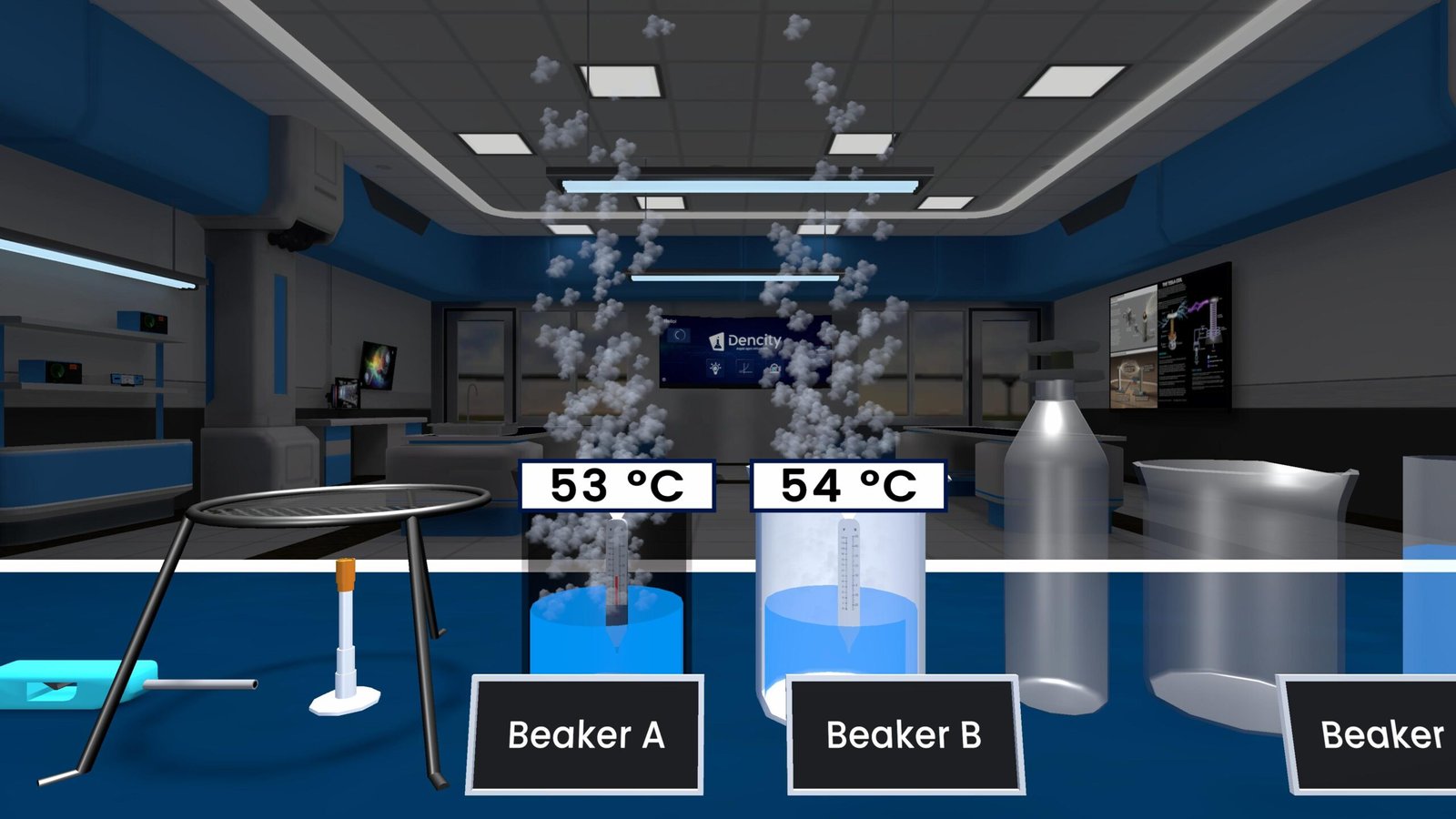
- 2 grams of lead nitrate (Pb(NO₃)₂) are heated in a boiling test tube.
- As it heats up, the compound decomposes into three products:
- Lead oxide (PbO): Yellow solid
- Nitrogen dioxide (NO₂): Brown toxic gas
- Oxygen (O₂): Colorless gas
The appearance of brown fumes confirms the release of NO₂ gas. This type of chemical breakdown under heat is called a thermal decomposition reaction.
Chemical Equation
2 Pb(NO₃)₂ (solid) → 2 PbO (solid) + 4 NO₂ (gas) + O₂ (gas)
This reaction illustrates how a single reactant splits into multiple products when heat is applied.
Real-Life Applications
- Metal extraction: Thermal decomposition of metal ores releases usable metals.
- Cement and lime industries: Decomposition of calcium carbonate is a key step.
- Car airbags: Use rapid decomposition of sodium azide to inflate airbags with nitrogen gas.
Observations from the Experiment
- Brown fumes of nitrogen dioxide (NO₂) are visible.
- A yellow residue of lead oxide (PbO) remains in the test tube.
- The reaction requires heat to begin, showing it is endothermic.
Reaction Table
| Reactant | Products | Type of Reaction |
|---|---|---|
| Pb(NO₃)₂ | PbO + NO₂ + O₂ | Decomposition |
Explanation of Products
- PbO (Lead(II) Oxide): Yellow solid that remains after heating.
- NO₂ (Nitrogen Dioxide): Toxic reddish-brown gas forming the visible fumes.
- O₂ (Oxygen Gas): Invisible gas released during decomposition.
Explore Decomposition Reactions with Dencity
With the Dencity virtual lab, students can perform this experiment safely and interactively. Heat compounds like lead nitrate virtually and observe color changes, gas emissions, and byproducts.
This is part of the Class 10 Science curriculum and is available in the Dencity app on Android, iOS, and desktop platforms.
Students can tweak variables, visualize changes, and get real-time feedback—making chemistry more understandable and fun.
Dencity for Teachers
Dencity enhances interactive teaching by offering:
- Virtual simulations for safe chemical reactions.
- Assignments with instant results and explanations.
- Tools to demonstrate reactions and track student progress.
- Collaborative virtual classrooms for hands-on learning.
Touch-Friendly for Interactive Panels
Dencity is fully compatible with interactive touch screens. Teachers and students can trigger reactions, change variables, and observe outcomes with simple touch gestures.
Request a Demo or Custom Pricing
Looking to bring virtual chemistry labs to your school? Contact us today for a free demo and custom pricing packages designed for educational institutions.
Frequently Asked Questions
- What is a decomposition reaction?
A single compound breaks into two or more simpler substances using energy. - Why is this reaction endothermic?
It requires heat to break chemical bonds. - What is PbO?
Lead(II) oxide, a yellow solid left behind after the reaction. - What causes the brown fumes?
The release of nitrogen dioxide gas (NO₂). - Is this safe to perform at home?
No, but it’s safely simulated in the Dencity app. - Which class is this for?
This is part of Class 10 Science. - Can teachers assign this virtually?
Yes, with automatic tracking and grading in Dencity. - Does Dencity explain reactions step-by-step?
Absolutely! It shows every part of the reaction clearly. - Is it compatible with phones and tablets?
Yes, Dencity works on Android, iOS, and desktop platforms. - How can I implement Dencity in my school?
Contact us to book a demo and explore your options.