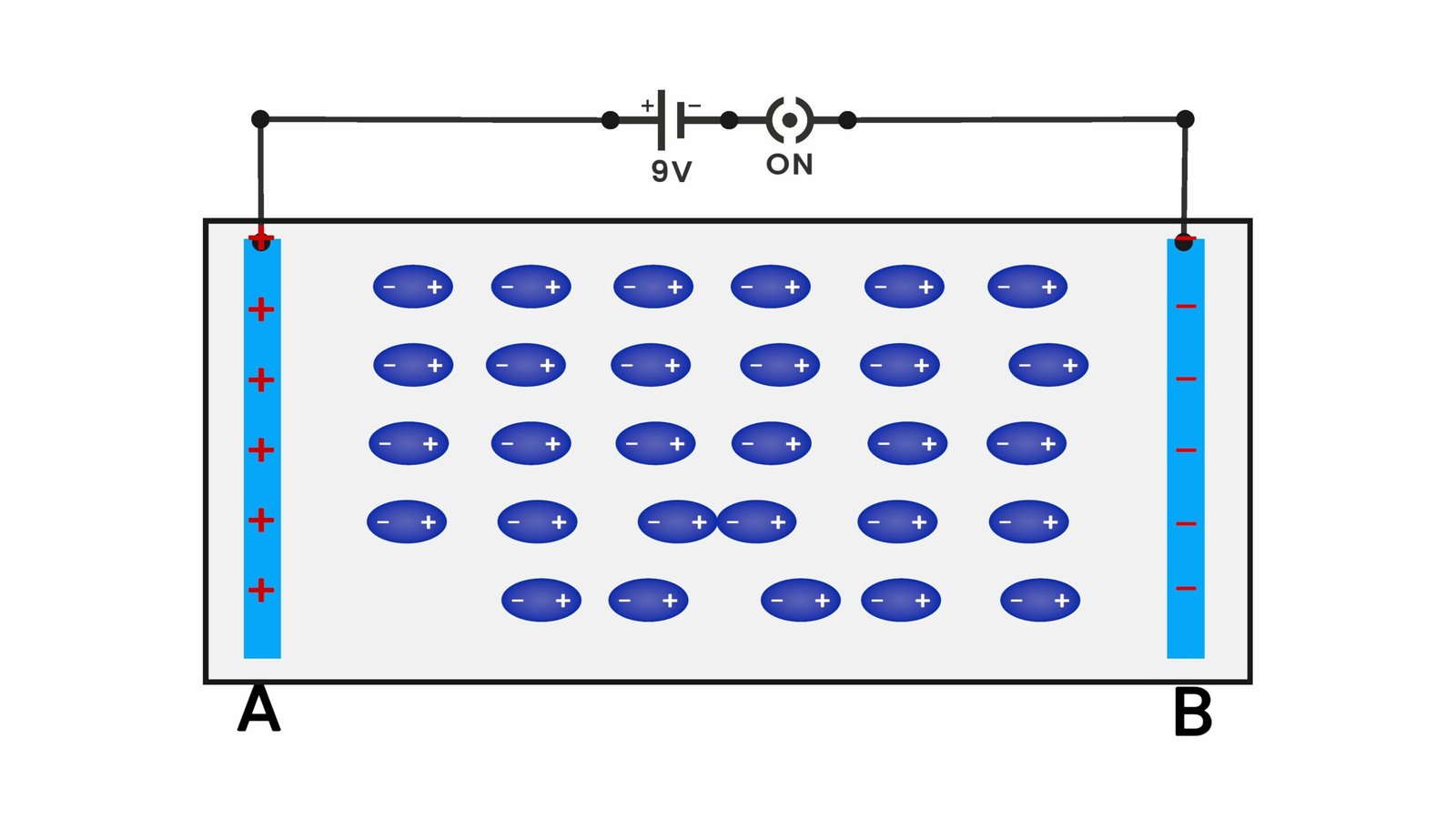
Polarity of Molecules – How Molecules Behave in Electric Fields
Class: class 12 science
What is Molecular Polarity?
The polarity of a molecule refers to how electric charges are distributed within it. A molecule is polar if the electrons are unevenly shared between atoms, creating a positive and negative end — like a tiny magnet. These molecules have a permanent dipole moment. On the other hand, non-polar molecules have their charge evenly balanced, so they don’t have a natural dipole.
Theory Behind Polar and Non-Polar Molecules
Molecular polarity is caused by differences in electronegativity (how strongly atoms attract electrons). In a polar molecule, the more electronegative atom pulls electrons closer, making one side slightly negative and the other slightly positive.
When a polar molecule is placed in an electric field, the dipole aligns with the field to reduce its potential energy. This is a natural behavior — systems always try to become more stable. The energy of interaction is given by:
- U = -pE cos(θ)
Where:
U = potential energy
p = dipole moment
E = electric field strength
θ = angle between dipole and field direction - When the molecule is perfectly aligned (θ = 0°) with the field,
U = -pE (minimum energy) - When the molecule is opposite (θ = 180°),
U = +pE (maximum energy)
For non-polar molecules, there’s no permanent dipole. But an external electric field can distort their electron clouds, creating induced dipoles. These also respond to electric fields, but their effect is weaker and slower.
Real-Life Uses of Polarity
- Water (polar solvent) can dissolve salts and other ionic substances due to its strong dipole.
- Non-polar solvents (like hexane) dissolve oils and fats better.
- Capacitors in electronics use dielectric materials (polar or non-polar) to store electric energy.
- In biology, polarity helps proteins fold correctly and form stable membranes.
Key Observations in the Experiment
- Higher voltage causes polar molecules to rotate and align faster.
- Lower voltage causes slower rotation.
- For non-polar molecules:
- At high voltage, they stretch more noticeably (due to stronger induced dipoles).
- At low voltage, the stretching is slower and harder to notice.
Summary Table
| Molecule Type | Electric Field Effect | Behavior Observed |
|---|---|---|
| Polar | Permanent dipole aligns | Rotates toward field direction |
| Non-Polar | Induced dipole forms | Stretches, but doesn’t align |
Case-wise Behavior Table
| Case | Observation |
|---|---|
| Polar molecules at low voltage | Rotate slowly to align with the field |
| Polar molecules at high voltage | Rotate quickly and align strongly |
| Non-polar molecules at low voltage | Stretch slowly due to weak induced dipoles |
| Non-polar molecules at high voltage | Stretch quickly due to strong induced dipoles |
Visualize Molecular Polarity with Dencity Virtual Science Lab
The Dencity app helps students see and understand molecular polarity like never before. With high-quality 3D simulations and real-time calculations, students can:
- Simulate the behavior of polar and non-polar molecules in electric fields.
- Change voltage and observe how molecules rotate or stretch in response.
- See how energy changes with the angle between the molecule and the field.
- Understand dipole moments, induced charges, and energy minimization interactively.
This experiment is part of the class 12 science curriculum and builds a strong foundation in electrostatics and molecular chemistry.
Dencity for Teachers
Dencity enables interactive teaching for advanced science topics:
- Visualize dipoles and molecular motion during live classes.
- Assign experiments and track how students adjust variables like electric field strength.
- Use interactive simulations to explain concepts like dipole energy, alignment, and induced dipoles.
- Replace abstract diagrams with real-time 3D behavior of molecules.
- Encourage interactive learning and critical thinking in class.
Optimized for Interactive Touch Panels
Dencity runs smoothly on interactive classroom panels, letting teachers:
- Zoom, rotate, and move molecules on-screen.
- Control the electric field strength using simple gestures.
- Show how molecule alignment changes dynamically — making science tangible and fun.
Contact Us for Demos and School Plans
Interested in making molecular concepts come alive in your classroom?
Visit dencityapp.in to book a free demo or ask for a custom pricing plan for your school.
Frequently Asked Questions (FAQs)
- What is a polar molecule?
A molecule with uneven charge distribution, creating a dipole. - Why do polar molecules align in electric fields?
Because the dipole interacts with the field, rotating to lower potential energy. - Do non-polar molecules react to electric fields?
Yes, they form induced dipoles and stretch slightly, though much less than polar molecules. - What does the formula U = -pE cos θ mean?
It gives the potential energy of a dipole in an electric field, depending on its angle to the field. - Why is water a good solvent?
Water is polar, so it dissolves ionic and polar compounds by attracting opposite charges. - What happens when voltage increases?
Molecules rotate or stretch faster and stronger due to increased electric force. - Can we simulate this in Dencity?
Yes, Dencity allows full simulation of dipole behavior and energy variation. - How does this help in real-world applications?
Concepts of polarity are used in electronics, biology, and chemical synthesis. - Is this suitable for class 12 science students?
Yes, it directly aligns with electrostatics and molecular chemistry topics. - Where can I get the Dencity app?
Visit dencityapp.in to access the app and explore its features.
Explore Polarity and Electric Fields Safely with the Dencity Virtual Science Lab — Where Science Comes to Life.