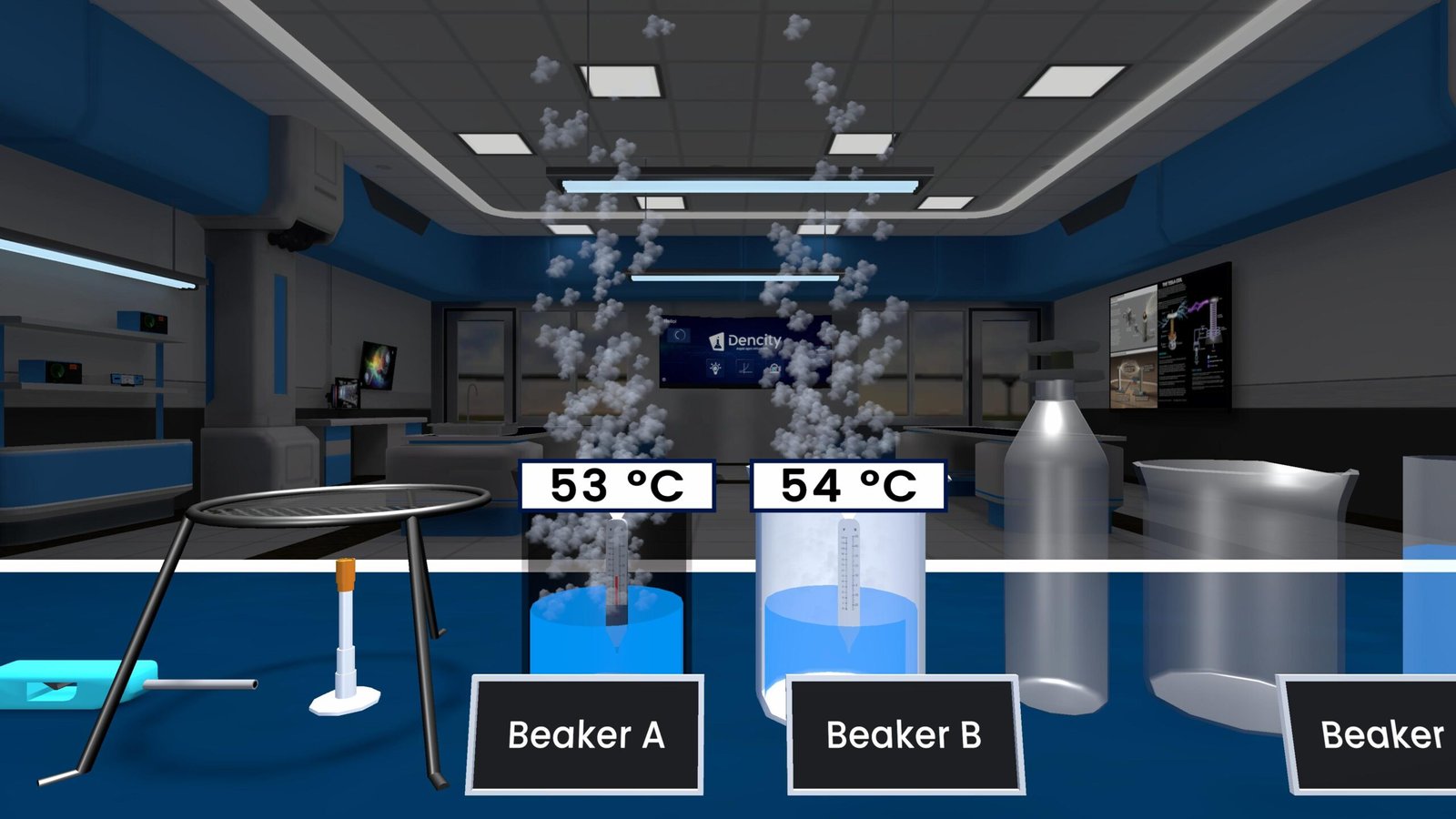
Displacement Reaction Experiment – Explained for Class 10 Science
A displacement reaction is a type of chemical reaction where a more reactive metal displaces a less reactive metal from its salt solution. This reaction type clearly shows the reactivity trend in metals and is an essential concept in Class 10 science.
Understanding Displacement Reaction
In simple terms, when a metal that’s more eager to react (more reactive) meets a solution containing a metal that is less eager (less reactive), the more reactive metal “pushes out” the less reactive metal from the solution. This happens because more reactive metals form compounds more easily.
Real-Life Example of Displacement Reaction
This experiment demonstrates the concept through two parts:
- Iron nail in copper sulphate solution:
- When you put an iron nail into blue copper sulphate solution, a reaction happens.
- Iron is more reactive than copper, so it kicks copper out of the solution and takes its place.
- The reaction is:
- Iron (Fe) + Copper Sulphate (CuSO₄) → Iron Sulphate (FeSO₄) + Copper (Cu)
- You will see brown copper deposit on the iron nail and the solution turns from blue to pale green due to iron sulphate.
- Copper wire in iron sulphate solution:
- When a copper wire is dipped into green iron(II) sulphate solution, no reaction occurs.
- This is because copper is less reactive than iron and can’t replace it in the solution.
Materials Used
- Iron nail (Fe) – Grey solid
- Copper wire (Cu) – Reddish-brown solid
- Copper sulphate solution (CuSO₄) – Blue colored
- Iron(II) sulphate solution (FeSO₄) – Green colored
What You Observe
- A brown layer of copper forms on the iron nail.
- The blue solution of copper sulphate turns pale green.
- No visible change occurs when copper is added to iron(II) sulphate.
Type of Reaction
This is a redox reaction, which shows how one metal gains and another loses electrons. It also teaches about the reactivity series – an important concept in chemistry.
Performing This Experiment Safely with Dencity
Want to try this experiment without dealing with real chemicals? The Dencity virtual lab lets students from class 9 to class 12 perform this experiment safely, virtually, and at their own pace.
The Dencity app is a virtual science lab available on Android, iOS, and Desktop. It helps students visualize science experiments like displacement reactions, understand them better, and explore “what if” scenarios without worrying about safety or cost.
Dencity for Teachers
For teachers, Dencity offers a revolutionary interactive teaching experience. You can:
- Create virtual classrooms
- Assign experiments as homework
- Track student performance automatically
- Let students control virtual lab setups in real time
This boosts interactive learning, saves classroom resources, and improves engagement through visual and practical exposure to theoretical concepts.
Dencity on Touch Panels
Dencity is fully optimized for interactive touch panels, turning traditional classrooms into digital science labs. Teachers and students can explore reactions, adjust variables, and conduct full experiments with simple touch gestures.
Get a Demo or Customized Plan
If you’re a school or educational institution, reach out to us for a free demo or to discuss custom pricing plans tailored to your needs.
Frequently Asked Questions
- What is a displacement reaction?
A reaction where a more reactive metal replaces a less reactive metal from its salt solution. - Why does iron displace copper in copper sulphate?
Because iron is higher in the reactivity series than copper. - Why doesn’t copper displace iron from iron sulphate?
Copper is less reactive than iron, so it cannot displace it. - What color change happens in the iron and copper sulphate reaction?
The blue solution turns pale green due to iron(II) sulphate formation. - Is this experiment safe to do at home?
It involves chemicals, so it’s best to do it virtually using the Dencity app. - Which class is this experiment part of?
This is usually covered in Class 10 science curriculum. - Is this a redox reaction?
Yes, one metal is oxidized and the other is reduced. - What does the brown layer on iron indicate?
It’s deposited copper, showing the reaction has occurred. - Can I try different metal combinations in Dencity?
Yes, the app allows multiple setups to explore reactivity series fully. - Does Dencity provide explanations?
Absolutely. It gives step-by-step solutions and real-time feedback for every experiment.
This content can be directly pasted into a WordPress post or page to educate students and promote the Dencity app effectively. Let me know if you’d like to prepare the next experiment in the same way!