Respiration Experiment (Class 9 Science & Class 10 Science)
Introduction & Concept
Respiration is the biochemical process by which cells convert glucose and oxygen into usable energy (ATP). During this process, carbon dioxide and water are released as by-products. Studying respiration helps us understand how living organisms obtain energy for growth, movement, and all vital functions.
Experiment Details
Objective: To demonstrate the higher concentration of carbon dioxide in exhaled air compared to ambient air using lime water.
Materials
- Two clean test tubes
- Freshly prepared lime water (saturated calcium hydroxide solution)
- A syringe or pichkari
- Stopwatch or timer
Procedure
- Setup A (Exhaled Air):
- Pour lime water into Test Tube A.
- Have a person blow steadily through a glass tube or straw into the lime water for a fixed duration.
- Start the timer as they begin to blow.
- Setup B (Ambient Air):
- Pour fresh lime water into Test Tube B.
- Using a syringe or pichkari, draw in ambient air and bubble it through the lime water at the same rate.
- Time how long it takes to observe any change.
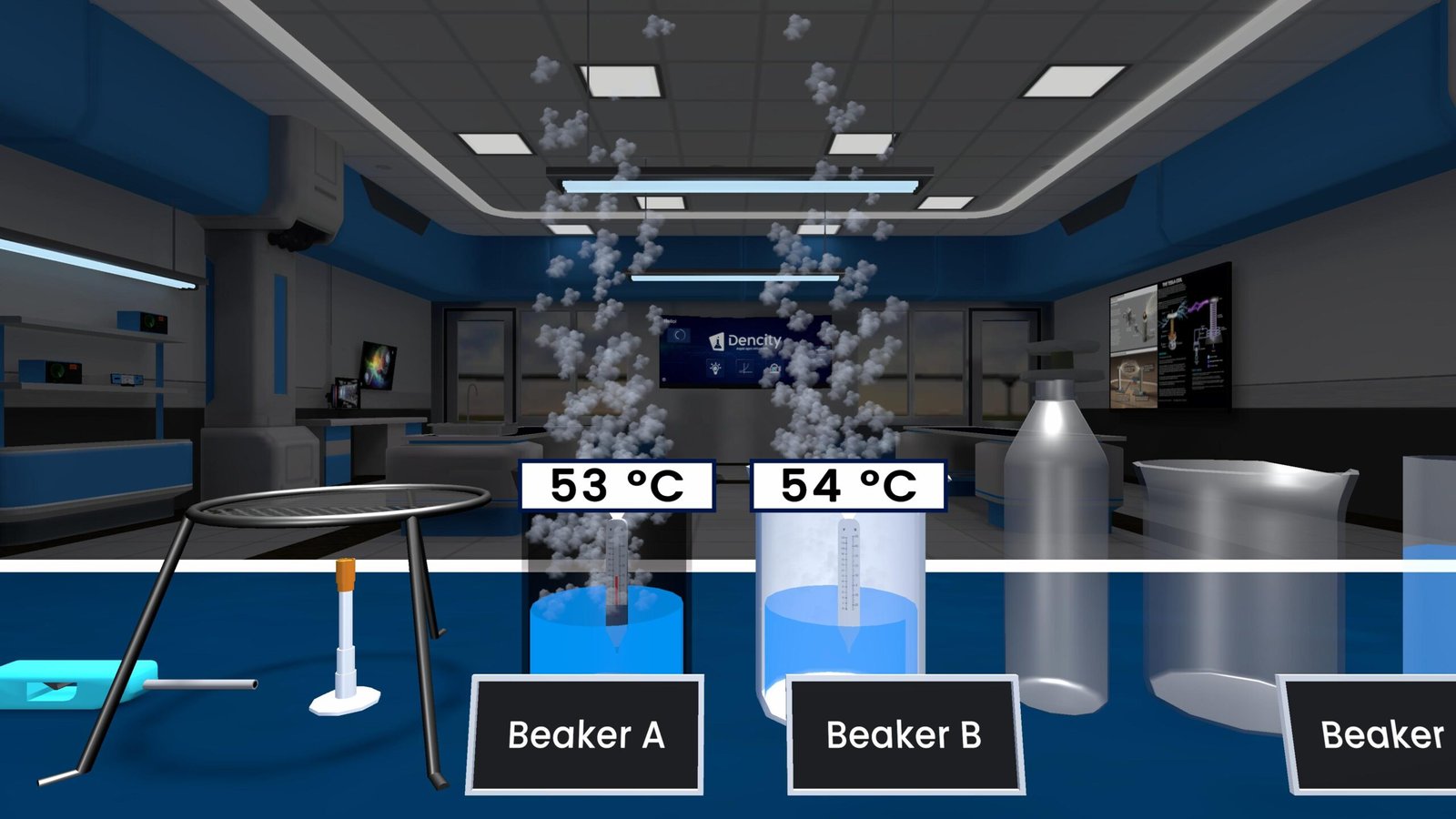
Observations
- Test Tube A: Lime water turns milky in approximately 15 seconds, indicating the rapid formation of calcium carbonate due to high CO₂ concentration.
- Test Tube B: Lime water turns milky more slowly (around 45–60 seconds), showing a lower CO₂ concentration in ambient air.
| Tube | Air Source | Time to Turn Milky | CO₂ Level |
|---|---|---|---|
| A | Exhaled Air | 15 s | High |
| B | Ambient Air | 45–60 s | Low |
Chemical Reaction
Calcium hydroxide (lime water) reacts with carbon dioxide to form calcium carbonate (insoluble) and water:
Ca(OH)₂ + CO₂ → CaCO₃ ↓ + H₂O
Real-Life Applications
- Respiration tests in biology to confirm CO₂ exhalation.
- Greenhouse gas studies, monitoring CO₂ exchange in plants.
- Medical diagnostics, measuring exhaled CO₂ to assess respiratory health.
Detailed Explanation
Respiration occurs in all living cells, from simple bacteria to complex multicellular organisms. It consists of two main stages:
- Glycolysis – Glucose breaks down into pyruvate in the cytoplasm, yielding ATP and NADH.
- Aerobic Respiration – In the mitochondria, pyruvate enters the Krebs cycle and electron transport chain, producing the bulk of ATP and releasing CO₂ and H₂O.
By comparing how quickly lime water turns milky in the two setups, students directly observe that exhaled air contains significantly more CO₂ than ambient air, proving the role of respiration in gas exchange.
Dencity & Virtual Science Lab
With the Dencity virtual lab, performing this Respiration Experiment becomes cost-efficient, safe, and highly interactive:
- Real-Time Simulation: Virtually bubble air samples through digital lime water and watch the turbidity change instantly.
- Step-by-Step Guidance: On-screen prompts guide students through each stage, reinforcing theoretical concepts with interactive learning.
- Data Logging: Automatically record time and CO₂ levels, exportable for reports.
- Repeatability: Run unlimited trials without consuming reagents or risking spills.
Dencity for Teachers
| Feature | Benefit |
|---|---|
| Interactive Teaching | Assign control of the virtual experiment to students in real time, fostering interactive teaching and engagement. |
| Homework & Tracking | Create assignments on science experiments and monitor submissions instantly. |
| Virtual Classroom | Host live demos, discussions, and group problem-solving remotely. |
| Customizable Parameters | Adjust CO₂ concentration, bubble rate, and observation time on the fly. |
| Automated Assessment | Built-in quizzes and instant feedback save grading time. |
Dencity virtual lab is fully optimized for interactive touch panels in classrooms, enabling smooth gestures for adjusting flow rate, starting/stopping timers, and annotating observations.
For customized pricing or a live demo, educational institutions can contact us at info@dencityapp.in.
Frequently Asked Questions
- Why does lime water turn milky with CO₂?
Because calcium carbonate precipitates out of solution when CO₂ reacts with calcium hydroxide. - Can this experiment run without lime water?
No; lime water is specific for detecting CO₂ via precipitation. - What factors affect the rate of milky formation?
Bubble rate, CO₂ concentration, temperature, and lime water saturation. - Is this experiment safe for students?
Yes, especially in the virtual science lab, where there’s no contact with chemicals. - How does Dencity help with science lab management?
It eliminates reagent costs, reduces setup time, and prevents hazards. - Can I export data from the virtual experiment?
Yes—downloadable CSV reports include time stamps and CO₂ levels. - Does Dencity support offline use?
The dencity app works on Android, iOS, and Desktop; some features are available offline. - Which classes is this experiment suitable for?
It’s ideal for class 9 science and class 10 science curricula. - How realistic are the virtual simulations?
Dencity uses high-fidelity physics models to match real-world outcomes. - Can I integrate Dencity with my school’s LMS?
Yes—Dencity offers APIs for seamless integration with popular learning management systems.