Gaseous State – Explained for Students
The gaseous state is one of the fundamental states of matter, where the particles are much farther apart compared to solids and liquids. These particles move rapidly and randomly, which is why gases do not have a fixed shape or volume. Instead, they take the shape and completely fill the volume of their container.
Understanding the Gaseous State
In gases, particles have high kinetic energy. This means they are always in motion, colliding with each other and with the walls of the container. These collisions are responsible for the pressure exerted by gases. Since the space between gas particles is large, gases are:
- Highly compressible
- Have low density
- Can expand freely to fill any container
Due to their fast-moving particles, diffusion happens quickly in gases. This is why smells spread so fast in a room. The behavior of gases can be understood using the Kinetic Molecular Theory, which also leads to important gas laws such as:
- Boyle’s Law: At constant temperature, the pressure of a gas is inversely proportional to its volume.
- Charles’s Law: At constant pressure, the volume of a gas is directly proportional to its temperature.
When you increase the temperature, gas particles move even faster. Increasing pressure pushes the particles closer, reducing the volume.
Real-Life Uses of Gases
- Oxygen (O₂) cylinders are vital in hospitals.
- LPG and CNG are used as fuels in homes and vehicles.
- Carbon dioxide (CO₂) is used in fire extinguishers and fizzy drinks.
- Helium is used in balloons and airships because it is lighter than air.
Key Observations in Gases
- When you reduce the volume of a gas while keeping the number of particles constant, the pressure increases.
- Gases spread quickly due to rapid diffusion.
- Pressure is caused by gas particles colliding with the container walls.
Summary Table
| Property | Gases | Effect |
|---|---|---|
| Shape | No fixed shape | Fills container |
| Volume | Not fixed | Expands freely |
| Compressibility | High | Easy to compress |
| Diffusion | Fast | Spreads quickly |
| Pressure | Exerts on walls | Due to collisions |
How Dencity Helps You Learn the Gaseous State
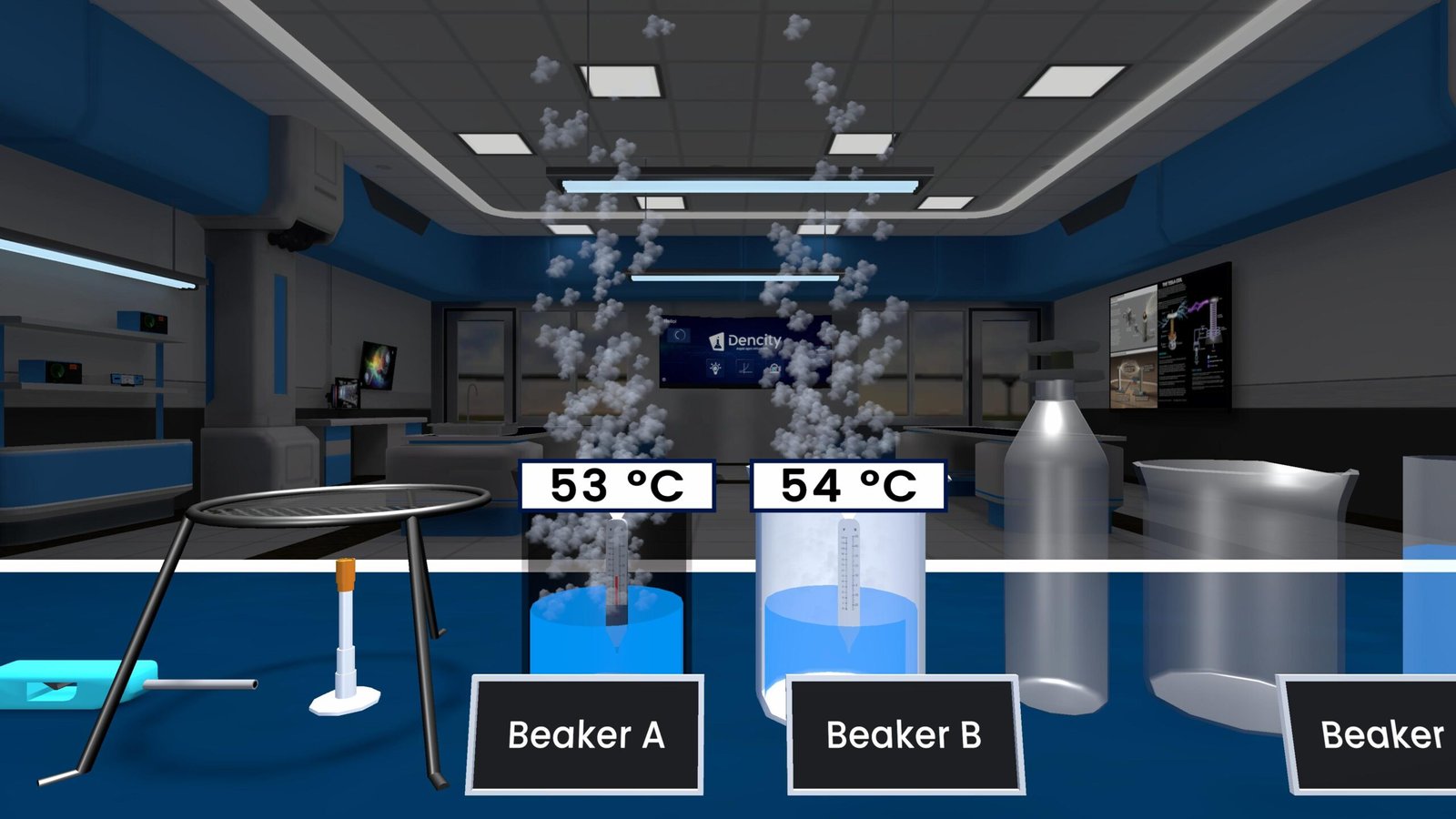
The Dencity virtual science lab lets students conduct Gaseous State experiments interactively. Whether you’re in class 9 science or higher, you can:
- Observe how gases behave under changing conditions.
- Simulate Boyle’s and Charles’s Law without needing gas cylinders or pressure gauges.
- Perform virtual science experiments safely and affordably.
- Visualize pressure changes, diffusion, and temperature effects in real-time with step-by-step calculations.
This makes it easier to understand how gases behave, using a science app that brings your textbooks to life!
Dencity for Teachers
Dencity empowers interactive teaching by allowing educators to:
- Use virtual classrooms to demonstrate gas behavior dynamically.
- Assign homework with virtual experiments in under 30 seconds.
- Track student progress and provide feedback instantly.
- Replace chalk-and-talk with interactive learning methods.
Optimized for Touch Panels
Dencity is designed to work seamlessly on interactive touch panels in classrooms. Teachers can manipulate gas particles, pressure, and temperature using simple touch gestures, making teaching engaging and fun.
Want Dencity in Your School?
Educational institutions can contact us for a customized pricing plan or a live demo of the Dencity virtual lab. Experience the future of science education today!
Frequently Asked Questions
- What is the gaseous state?
It is a state of matter where particles are far apart, move freely, and have no fixed shape or volume. - Why are gases compressible?
Because there is a lot of space between particles, allowing them to be pushed closer. - What causes gas pressure?
Collisions of gas particles with the walls of their container. - What is diffusion in gases?
The mixing of gas particles due to their random motion. - How do temperature changes affect gases?
Higher temperatures increase particle speed and volume. - What laws do gases obey?
Boyle’s Law and Charles’s Law. - Why do gases fill their container?
Because their particles move randomly and freely. - What are some real-life uses of gases?
Medical oxygen, LPG, CNG, helium balloons, CO₂ in sodas. - How does Dencity help in learning gases?
By providing virtual experiments and simulations of gas laws. - Can teachers use Dencity for live demos?
Yes, Dencity supports interactive classrooms and real-time control transfer.
Explore science the smart way with the Dencity app – your very own virtual science lab!